
Potassium Fluoride‐Catalyzed Hydroboration of Aldehydes and Ketones: Facile Reduction to Primary and Secondary Alcohols - Kuciński - 2020 - European Journal of Organic Chemistry - Wiley Online Library
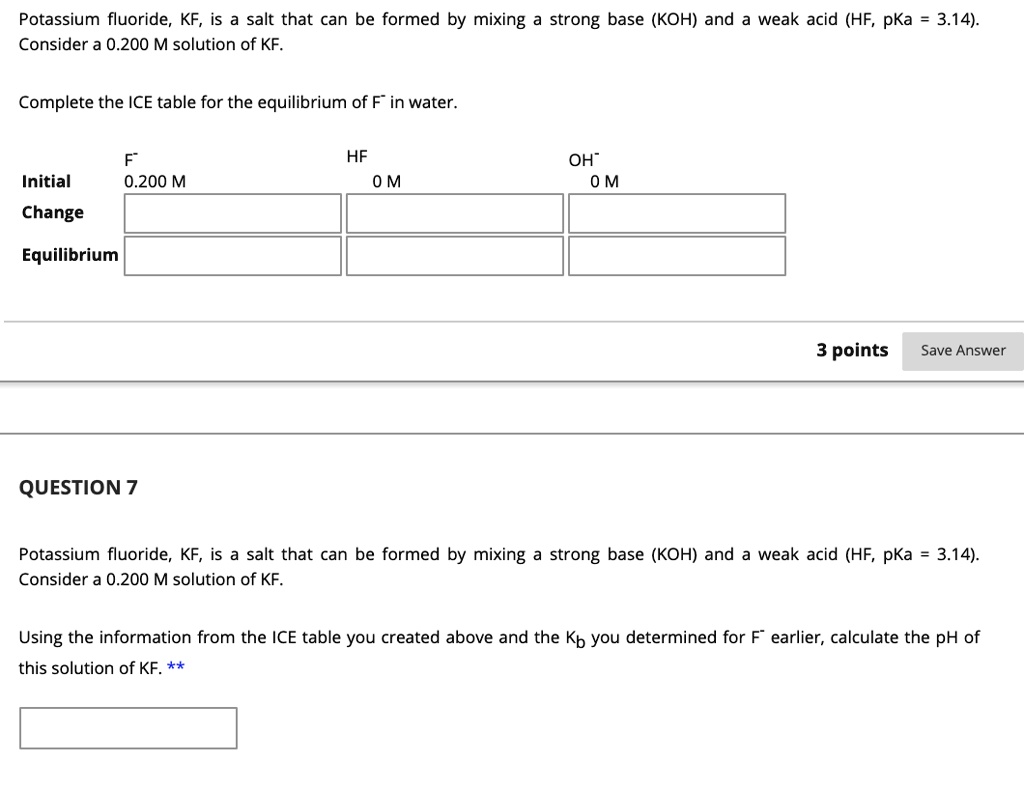
SOLVED: Potassium fluoride, KF, is salt that can be formed by mixing a strong base (KOH) and weak acid (HF, pKa 3.14). Consider a 0.200 M solution of KF. Complete the ICE
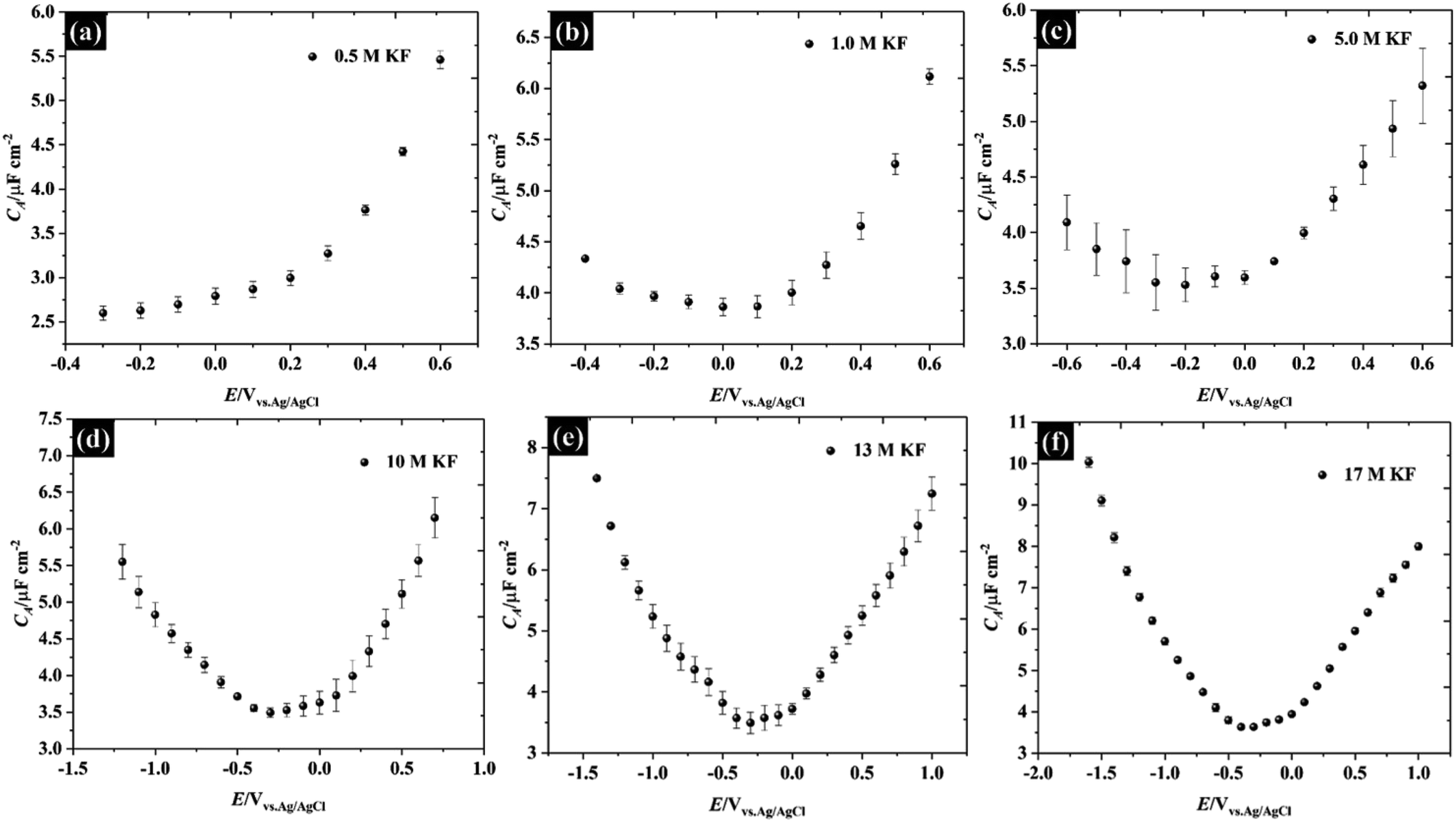
![Potassium fluoride activation for the nucleophilic fluorination reaction using 18-crown-6, [2.2.2]-cryptand, pentaethylene glycol and comparison with the new hydro-crown scaffold: a theoretical analysis - Organic & Biomolecular Chemistry (RSC Publishing) Potassium fluoride activation for the nucleophilic fluorination reaction using 18-crown-6, [2.2.2]-cryptand, pentaethylene glycol and comparison with the new hydro-crown scaffold: a theoretical analysis - Organic & Biomolecular Chemistry (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C8OB00418H)
Potassium fluoride activation for the nucleophilic fluorination reaction using 18-crown-6, [2.2.2]-cryptand, pentaethylene glycol and comparison with the new hydro-crown scaffold: a theoretical analysis - Organic & Biomolecular Chemistry (RSC Publishing)
















![Potassium Chloride [50lbs Bag] | Hydro-Gardens Potassium Chloride [50lbs Bag] | Hydro-Gardens](https://hydro-gardens.com/wp-content/uploads/2014/12/PotassiumChloride.jpg)